主な利点
-
エンドトキシンと汚染管理
-
正確に定量化された力価、ゲノムコピー、完全性
-
安全リスクを低減し、有効性を高める
-
再現可能な結果
サービスの詳細
| AAV type | Scale | Turnaround time |
|---|---|---|
| Standard capsids (guaranteed yield) | 1E+13 GC~8E+15 GC | Start from 17 business days |
| Custom capsids (by volume) | Up to 100L with 30mL-200mL small scale test | Start from 25 business days |
AAV の収量は、血清型や目的遺伝子といった要因によって異なります。収量の多い血清型(1、3b、5、7、8、9、Rh10)については、保証付きのパッケージを提供しています。その他の血清型については、容量に基づいて生産し、必要な生産量を決定するための小スケールテストを実施できます。詳細については技術サポートにお問い合わせください。
品質管理基準(QC Standards)
NHP グレードの AAV は、出荷前に厳格な QC 試験を経て、AAV 製品の品質を保証し、動物実験における副作用を最小限に抑えます。
| Test | Method | QC Standard | |
|---|---|---|---|
| Standard QC | ddPCR | Measure titer, normalized to 1e13vg/ml | Standard capsids: Concentration and total quantity meet needs. Custom capsids: quantity based on production scale. |
| AAV Capsid size* | SDS-PAGE silver stain | Match capsid protein size | |
| Guarantee endotoxin | LAL | <1EU/ml | |
| Mycoplasma Detection | qPCR | Negative | |
| Bioburden | Direct inoculation | No growth | |
| AAV Genome integrity | CE(titer>1e+12vg/ml, volume >50ul) | Report | |
| Empty Capsid Rate | TEM | <20% | |
| Additional QC | AAV Genome sequencing | Nanopore | Report |
| Empty Capsid Rate | AUC | Report | |
| Endotoxin removal | LAL | <0.2EU/ml | |
| Residual Triton Analysis (bundle with endo removal) | HPLC | 5ppm | |
| Sterility test | Direct inoculation | No growth |
パフォーマンス
-
AAV の品質、純度、安全性に関する包括的な試験を実施し、NHP(非ヒト霊長類)研究への適合性を保証
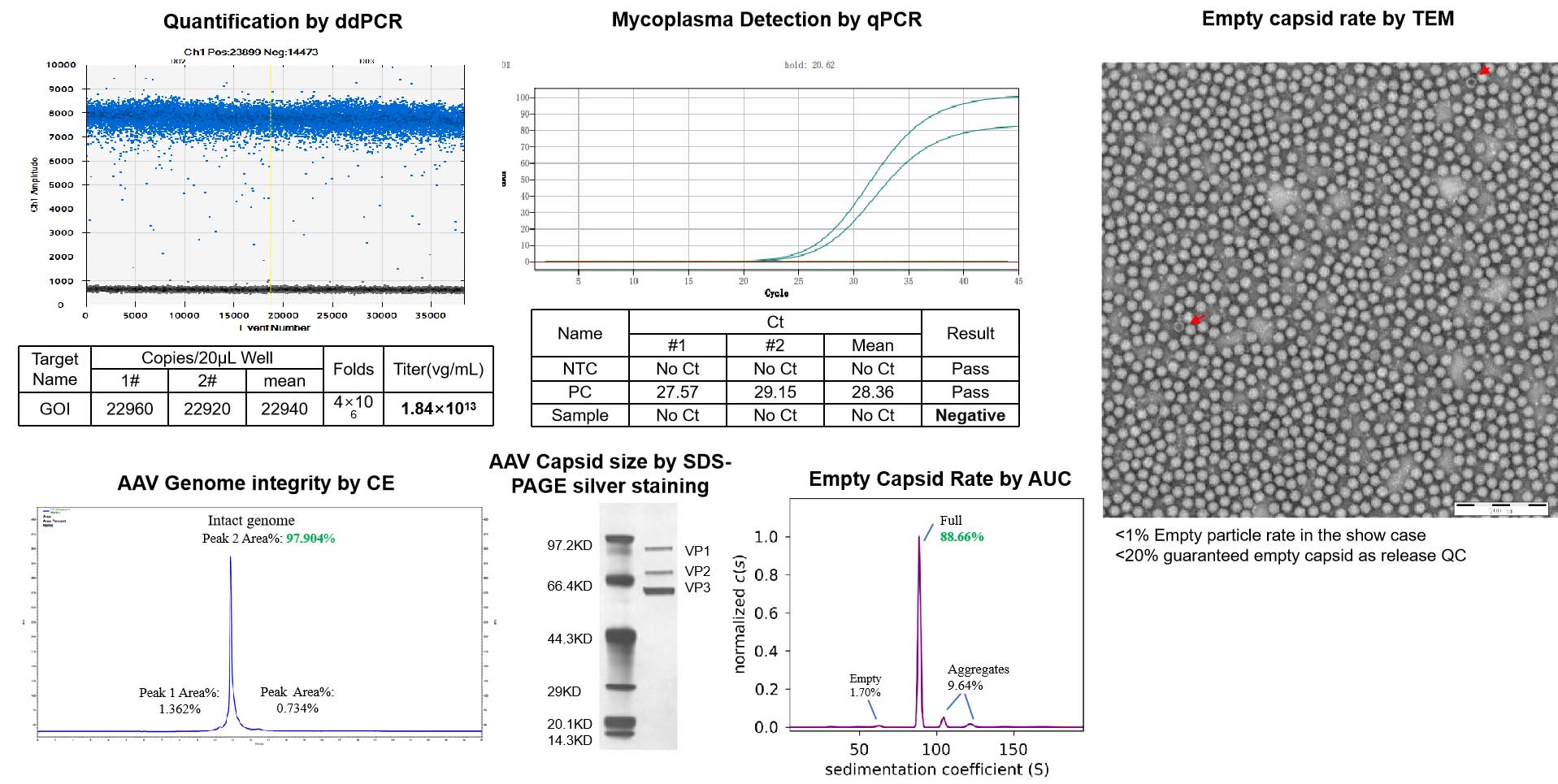
-
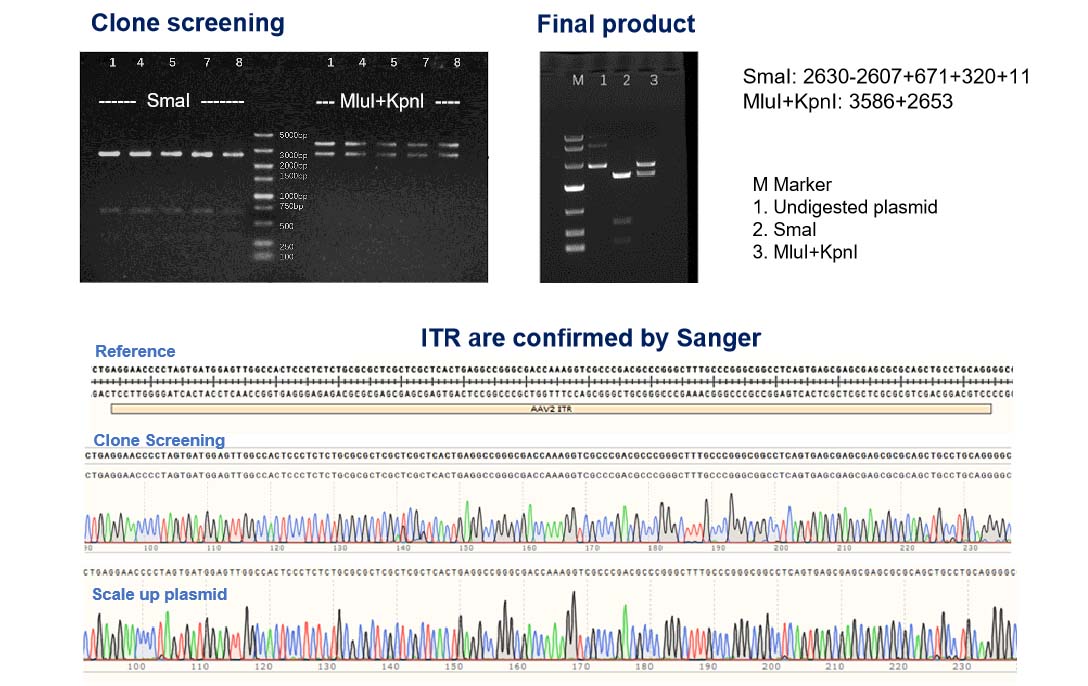
プラスミドの完全性を保証:高品質な NHP グレード AAV 生産のための ITRと GOI(目的遺伝子)の二重チェック